WHAT A DIFFERENCE
A SPRAY CAN MAKE
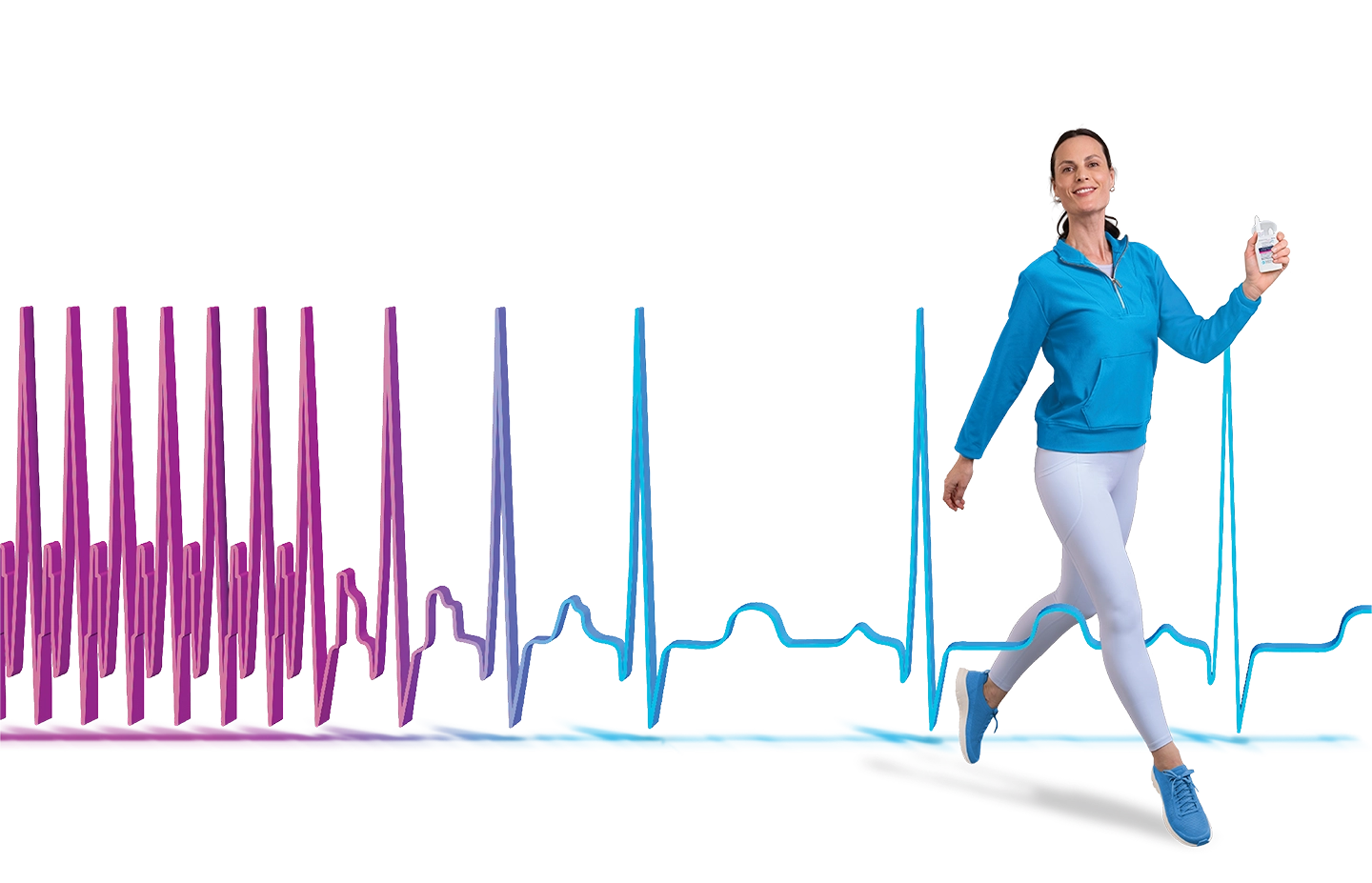
CARDAMYST™ (etripamil) is the first and only FDA-approved nasal spray approved to treat PSVT in adults, and rapidly restored sinus rhythm with a 17-minute median time to conversion1,2*
Conversion to sinus rhythm occurred within 30 minutes during confirmed paroxysmal supraventricular tachycardia (PSVT) episodes in 64% of patients with CARDAMYST vs 31% with placebo. Median time to conversion was 17.2 minutes with CARDAMYST vs 53.5 minutes with placebo.1
CARDAMYST was studied in a randomized, double-blind, placebo-controlled, multicenter, event-driven, phase 3 study to evaluate its efficacy and safety when self-administered in patients with a history of symptomatic PSVT. The primary endpoint was time-to-conversion of confirmed PSVT to sinus rhythm for at least 30 seconds within 30 minutes of the first dose. Of the episodes reviewed, 184 were confirmed by blinded adjudication to be PSVT and were included in the primary analysis.1
Product shown is not actual size

Product shown is not actual size
CARDAMYST prescriptions should read as follows1,3*:
Medication: CARDAMYST (etripamil) nasal spray 70 mg, 2 device pack
Sig: Use 1 spray in each nostril. If symptoms continue after 10 minutes, repeat using a second device
Dispense: 1 carton containing 2 devices
Refills: 3
Sample Rx only, based on recommended dosage in CARDAMYST Prescribing Information.
How CARDAMYST is supplied
2 devices
CARDAMYST is supplied as 2 disposable nasal spray devices contained in a plastic carrying case. One dose of CARDAMYST is 2 sprays, 1 spray for each nostril.1
Learn more about administering CARDAMYST
This is the Device Demonstration Video for the CARDAMYST™ (etripamil) nasal spray.
It is for intranasal use only.
Please read the full Patient Information and Instructions for Use contained in the medication carton before administering the medication.
If you have any questions concerning the use of CARDAMYST call 1-844-805-5810 or visit www.cardamyst.com.
Important Information You Need to Know Before Using CARDAMYST
For use in the nose only.
Each CARDAMYST device contains 1 dose consisting of 2 sprays (1 spray in each nostril).
Do not press the CARDAMYST device plunger until you are ready to deliver a dose.
Check the expiration date before using.
Do not test or prime before use.
The nasal spray device is ready for use.
CARDAMYST may cause dizziness.
Use CARDAMYST while sitting in a safe area where you will not fall if you become dizzy or lightheaded.
If you do not get the full first dose, wait 10 minutes before using another device.
Do not re-use the CARDAMYST device. Throw away the CARDAMYST device after use.
Step one: preparing to dose.
Each case contains two CARDAMYST devices.
One device contains one dose consisting of two sprays. One spray for each nostril.
Open the lid and carefully remove one device from the carrying case.
Step two: using the CARDAMYST nasal spray device.
Sit down with the CARDAMYST device in your hand.
Hold the device in an upright position, as shown with your thumb on the bottom of the plunger and the tip between your index and middle finger.
Do not press the plunger yet.
Step three: breathe normally and keep your head straight.
Insert the tip of the device into one nostril until your index and middle finger are touching the bottom of your nose.
Using your thumb, press the plunger firmly and quickly all the way up.
Continue to breathe normally and avoid inhaling deeply.
Step four: completely release the plunger and remove the device from the first nostril.
Step five: with the same device, immediately insert the tip of the device into the second nostril until your index and middle finger are touching the bottom of your nose.
Breathe normally and keep your head straight.
Using your thumb, press the plunger firmly and quickly all the way up.
Continue to breathe normally and avoid inhaling deeply.
Next, completely release the plunger and remove the device from the second nostril.
Step six: stay seated and keep your head straight for 10 minutes.
For CARDAMYST to work right away, keep your head straight for 10 minutes, as shown.
When medicine drips out, wipe your nose.
Do not blow your nose for 10 minutes.
Throw away the CARDAMYST device after use.
Step seven: if you continue to have symptoms after ten minutes, repeat steps 1 to 6 with a new CARDAMYST device.
If your symptoms have not improved within 20 minutes after your second dose, call your healthcare provider or get emergency medical help right away.
Do not use more than 2 CARDAMYST devices within a 24-hour period.
Replace the used CARDAMYST device with a new one right after use, so you will have a new nasal spray device in case you need it.
When storing CARDAMYST, store at 68°F to 77°F.
Make sure to keep CARDAMYST and all medicines out of reach of children.
IMPORTANT SAFETY INFORMATION FOR CARDAMYST™ (etripamil)
What is CARDAMYST?
CARDAMYST is a prescription medicine used to help restore normal heart rhythm in adults who have symptoms of sudden episodes of fast heartbeat called paroxysmal supraventricular tachycardia (PSVT).
It is not known if CARDAMYST is safe and effective in children.
Do not use CARDAMYST if you:
are allergic to CARDAMYST or to any of its ingredients. See the end of the Patient Information document for a complete list of ingredients in CARDAMYST.
have limitations in activities due to heart failure (moderate to severe heart failure).
have Wolff-Parkinson-White (WPW) syndrome, Lown-Ganong-Levine (LGL) syndrome, or an abnormal heart rhythm pattern called pre-excitation (delta wave) on an electrocardiogram (ECG).
have sick sinus syndrome without a permanent pacemaker.
have second degree or higher atrioventricular (AV) block.
Before using CARDAMYST, tell your healthcare provider about all of your medical conditions, including if you:
have a history of fainting.
have low blood pressure.
are pregnant or plan to become pregnant. It is not known if CARDAMYST will harm your unborn baby.
are breastfeeding or plan to breastfeed. It is not known if CARDAMYST passes into your breast milk. You should stop breastfeeding for 12 hours after treatment with CARDAMYST. During this time, pump and throw away your breast milk. Talk to your healthcare provider about the best way to feed your baby after using CARDAMYST.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What are the possible side effects of CARDAMYST?
CARDAMYST may cause serious side effects, including:
Fainting due to CARDAMYST effects on blood pressure, heart rate, and electrical activity of the heart. CARDAMYST may cause dizziness and fainting, especially in people with a history of fainting and certain heart problems, or people with a history of fainting during an episode of PSVT. Use CARDAMYST while sitting in a safe area where you will not fall if you become dizzy or lightheaded. Lie down if you feel dizzy or lightheaded after using CARDAMYST. If fainting occurs after using CARDAMYST, caregivers should place you on your back and seek medical help.
The most common side effects of CARDAMYST include:
nasal discomfort
nasal congestion
runny nose
throat irritation
nosebleed
These are not all of the possible side effects of CARDAMYST.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Please see the accompanying full Prescribing Information, including Patient Information.
CARDAMYST reimbursement support
Information to support your office when navigating Prior Authorizations and Medical Exceptions
Important information for managing a medical exception directly
Use these details to help ensure that eligible patients receive timely access to CARDAMYST.
Drug Name
CARDAMYST (etripamil) nasal spray1
Indication
CARDAMYST is indicated for the conversion of acute symptomatic episodes of paroxysmal supraventricular tachycardia (PSVT) to sinus rhythm in adults1
Dosage Form and Strength
Nasal spray, 70 mg of CARDAMYST per device1
Route of Administration
Nasal spray
Administration
- Initial dosage: A dose of 70 mg is administered as 2 nasal sprays, 1 spray into each nostril. Each nasal spray device delivers 2 sprays. The 2 sprays together contain a total of 70 mg of CARDAMYST1
- Repeat dosage (if needed): Should symptoms persist for 10 minutes after administration of CARDAMYST, take a second dose of 70 mg administered as 2 nasal sprays, 1 spray into each nostril. Do not exceed 140 mg in a 24-hour period1
ICD-10 Code
I47.1 Supraventricular tachycardia4
Diagnosis
Paroxysmal Supraventricular Tachycardia (PSVT)
Start the conversation about CARDAMYST with your patients.